The Smallest Solution to Large Problems
nanometer: one-billionth of a meter-the size at which biological molecules and structures inside living cells operate
nanometer: one-billionth of a meter-the size at which biological molecules and structures inside living cells operate
Working at the nanoscale level is showing promise for everything from vastly increased computer power to cancer treatments with fewer side effects. Yet in the medical field, a lab may develop the most amazing nanotherapy, but if it doesn't have a reliable way to manufacture large quantities approved for human use, the treatment will never be able to help anyone.
The Department of Defense is leading the way in creating a cGMP compliant nano manufacturing facility. Under FDA regulations, a facility producing and testing drugs and medical devices must comply with "current good manufacturing practices," or cGMP guidelines, to ensure quality. Many other countries have created similar GMP guidelines. There are yet very few such approved nanomedicine production centers in the world.
The U.S. Army Medical Research and Materiel Command's Telemedicine and Advanced Technology Research Center is supporting scientists in developing a facility at the University of Texas in Dallas. The center's first goal is to make nanoparticles targeted for lung and prostate tumor diagnosis and treatment. The hope is that the facility could adapt to manufacture a variety of human-grade nano agents.
Dr. Warren Grundfest, who manages TATRC's nanomedicine and biomaterials research portfolio, says, "A pharmaceutical-grade production facility is key in getting the latest science out to the soldier, and to the public. Through this and other projects, the military is leading the way in exploring nano drug action, toxicology, production ... basically, the potential and safety of nanoparticles. They could be the smallest solution to very large problems."
Pharmacology professor Dr. Jinming Gao of the University of Texas Southwestern Medical Center and engineering professor Dr. Wenchuang Hu of the University of Texas, Dallas, are creating the facility. Gao is working with UT Southwestern oncology professor Dr. James Willson to test the new nanoparticle delivery methods they have developed.
The team is one of the few working on an alternative "top-down" production process in which a precisely engineered stamp shapes material into nanoparticles, using techniques developed in the semiconductor industry. As opposed to most current nanomedicine approaches in which particles self assemble from the bottom up via chemical reactions, the top-down method provides better uniformity and the ability to make any shape of particle.
Recent research has found that the shape of nanoparticles makes a big difference in the effectiveness of the drug they are carrying. Nonspherical shapes remain in the blood longer and have more surface contact with target cells. The UT Southwestern and UT Dallas team is making nanorods of diagnostic materials and drugs for cancer.
Says Gao, "Our TATRC-managed project involves developing a facility with the flexibility to make rod-shaped nanoparticles. Once we have an FDA-approved facility and sufficient proof of positive medical impacts, we can begin testing in human patients, ideally within three to four years. If successful, we could then scale up for efficient manufacturing of these and many other shapes."
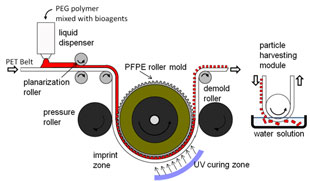
The team has designed a device much like a nanoscale inkjet printer. It uses a roll-to-roll printing process, similar to printing newspapers or labels. A one-inch-wide plastic web coated with a bioagent-filled polymer is continuously fed into a set of rollers. One roller acts as a stamp, pressing into the soft polymer on the web to form discrete nanoparticles. The patterned polymer particles are immediately solidified by exposure to ultraviolet light and released.
Says TATRC director Col. Karl Friedl, "Having a foundry to create a large amount of nanomaterials is a good idea. When promising nanoparticles come along to treat cancer or aid in wound healing, we want to be ready with the capability to manufacture them."
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.


