Saving Lives by Saving Blood
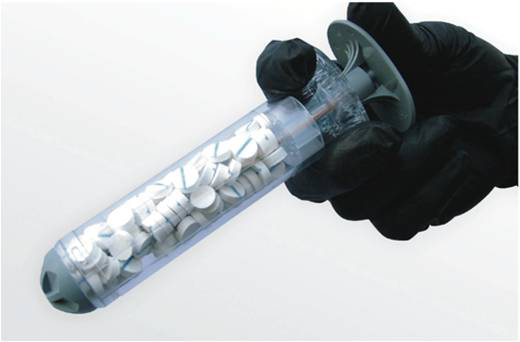
In an age of ultra high-tech devices that are worthy of screen time in a big budget Hollywood action movie, one might be surprised to discover that a very "unglamorous" and simplistic medical device is being developed by the Combat Casualty Care Research Program of the U.S. Army Medical Research and Materiel Command, Fort Detrick, Md., that may soon save lives on the battlefield and beyond.
"Hemorrhage is the leading cause of death on the battlefield, and one of our most challenging forms of hemorrhage has been junctional [the junction of the legs or arms with the torso] hemorrhage, or hemorrhage from deep wounds on which it is impossible to put a tourniquet or apply manual compression externally," said Dr. Anthony Pusateri, portfolio manager of the Department of Defense Hemorrhage and Resuscitation Research and Development Program, managed by the CCCRP.
Cue XSTAT ' the new device so practical, one will wonder how the medical field has not thought of it until now. Simply put, the device looks like a large plastic syringe filled with many small, pellet-shaped sponges that enlarge to fill up a wound area quickly to prevent blood loss.
"This XSTAT device allows the haemostatic material to be put [injected] into the wound tract, and then it expands from the inside out, putting pressure on the bleeding to stop it," said Pusateri. "It is a capability that has never existed before, and can be used in the field setting by medics, possibly even with buddy aid [first aid administered by battlefield 'battle buddies']."
As the portfolio manager, Pusateri must orchestrate matters among the various Services and the company in charge of production, RevMedx, that are collaborating for the successful development and release of this new medical device. However, as he states, Pusateri came into the project during its later development, at the time the Army took over management of the program.
"This project started with the United States Special Operations Command to conduct the initial work," he said. "The research looked promising enough that the Army chose to fund it through its completion."
While the current version of the device is useful for large wounds, Pusateri said that the next iteration will be one suited for narrower wound tracts. Due to the location of the target wounds, the original concept was for a common-size wound tract. As development progressed, the researchers found that in some cases, the wound tract is smaller, and it would not take much modification to create a smaller device to place into the wound. Pusateri said that the applicator will be narrower, although the sponge-like product inside will be the same size.
When asked about the timeframe for U.S. Food and Drug Administration approval, Pusateri said it should most likely receive approval during this summer.
"We have not identified any significant problems, and we expect it will fill a capability gap that we've had for quite some time," he said. "Upon FDA approval, the device will be commercially available off the shelf. I expect that RevMedx will do an initial production run."
Regarding military use, Pusateri said that he expects that units will be able to purchase the device for themselves. The device will receive a National Stock Number, which will allow any group to purchase the device for use. He also believes that the XSTAT device will transcend military medical use and find its way into the field of civilian medicine for widespread public use in trauma scenarios.
While the XSTAT device is certainly small and light enough to be carried in military aid bags or combat lifesaver bags, Pusateri said this does not guarantee its use as standard military medical equipment.
So why wouldn't the military want to have this in every warfighter's bag?
"Well, for one, this doesn't solve all of our bleeding problems," said Pusateri, "but it does fill a capability gap for use on a wound area where you could not place a bandage or tourniquet."
"It's primarily intended for deep wounds with heavy bleeding inside, with no way of putting direct pressure on the wound, and no way to wrap a tourniquet around it. Basically, it's designed for use in the axillary and inguinal regions, or the junction of the legs and arms with the torso ' too high for a tourniquet and too deep for a dressing."
Pusateri added, "Any new item must be considered along with all of the others items that medics, or others, must carry. Many factors come into play for the final fielding decision. In the Army, the Directorate of Combat and Doctrine Development at the U.S. Army Medical Department Center and School and the U.S. Army Medical Materiel Agency will play major roles in fielding decisions."
Despite its specificity ' or perhaps because of it ' the XSTAT device seems to be a necessary part of the future of both military and civilian medicine, and it is a perfect illustration of creating a medical item to satisfy a critical need in the field.
"This is a good example of very innovative and thorough work done by USSOCOM to get this started, working with the initial lead company [that later transitioned to RevMedx]," said Pusateri, "but it is also an example of excellent cooperation and communication in a joint environment, between USSOCOM and the U.S. Army."
"This could certainly be a significant advance in our ability to control hemorrhage, both on and off the battlefield," said Pusateri.
While the XSTAT device is currently on the forefront of the CCCRP's work, Pusateri said that his group continues to work on developing blood-related items to help save lives. On the horizon, the CCCRP is researching dry plasma products to help restore/replenish blood loss quickly and help make the body more resistant to the detrimental effects of severe bleeding.
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.


