USAISR Conducts FDA-Approval Clinical Trial for CRI
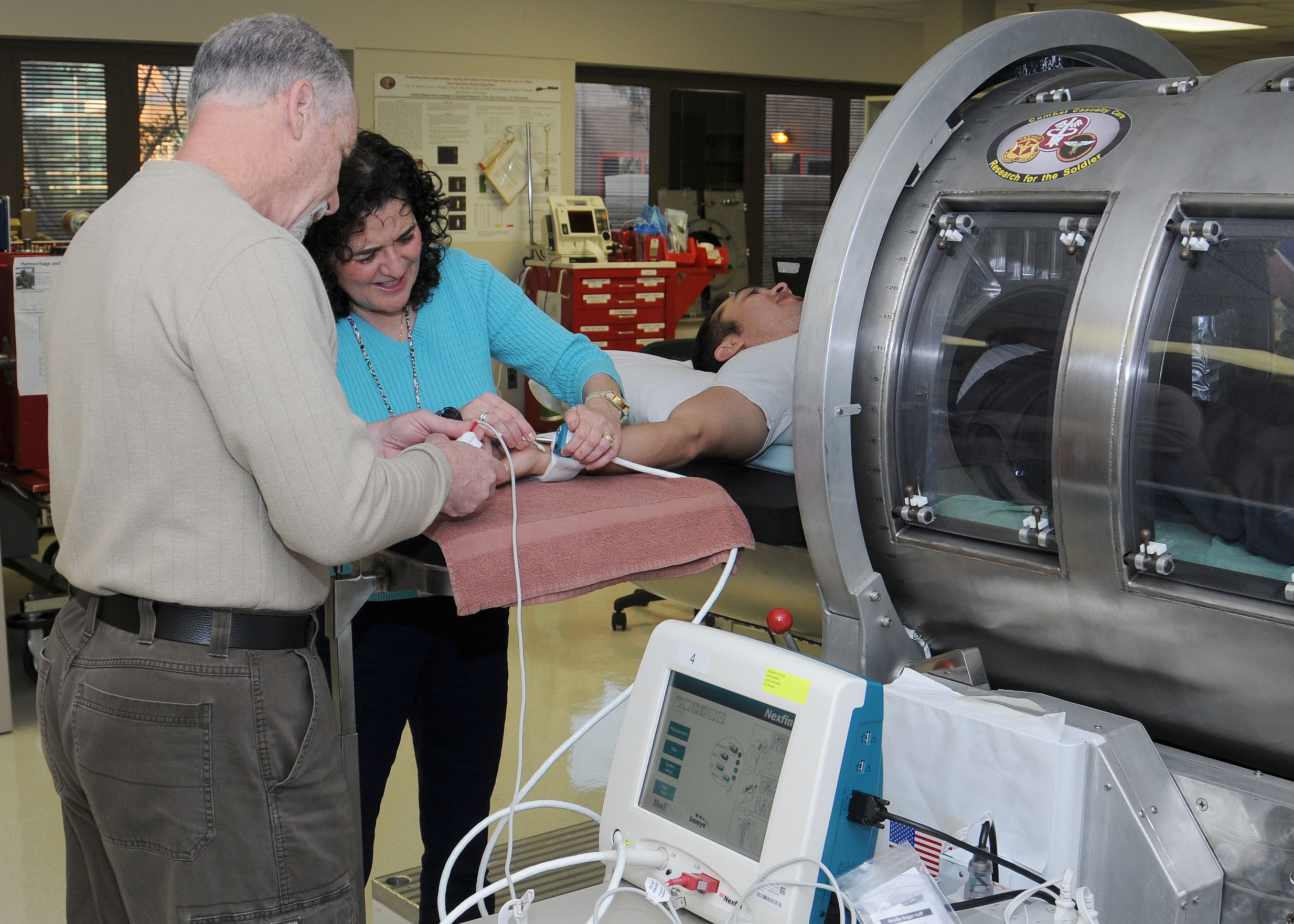
Researchers at the U.S. Army Institute of Surgical Research at Joint Base San Antonio-Fort Sam Houston, Texas are conducting a clinical trial on the Compensatory Reserve Index to gather data for submission to the Food and Drug Administration for 510(K) clearance.
Victor A. Convertino, Ph.D, at USAISR developed the CRI in collaboration with colleagues at the University of Colorado, Children's Hospital Denver, and Flashback Technologies Inc. The CRI uses an algorithm that is designed to take information from a patient using a non-invasive finger pulse oximeter and gauges whether immediate medical attention is needed even though the patient may seem alert and responsive.
The tactical combat casualty care research task area team led by Convertino will use a lower body negative pressure chamber during the clinical trial to gather the data. Research participants are placed in the LBNP chamber which draws their blood to their lower bodies.
"It's a way of 'bleeding' someone without taking a drop of blood," said Convertino. That's because the human body has many physiological mechanisms that compensate to maintain a constant blood pressure when there's internal bleeding.The blood pressure can seem stable, but the patient can be losing their ability to continue to compensate. When the patient gets to the end of their compensation, their blood pressure falls rapidly, referred to by some as 'falling off a cliff,' and now they are in shock," Convertino continued.
According to Convertino, medics, corpsmen and emergency medical service providers have traditionally been trained to watch a patient's blood pressure. The CRI algorithm can gauge how much the body is compensating and how much the body has left to compensate. This presents them with enhanced information regarding when a patient is in danger of going into shock.
In order to measure a patient's reserve to compensate, Convertino focused on an arterial waveform that is created by blood going out into the vessels.
"Each time the heart pumps, a pulse of blood creates an arterial pressure wave that is actually made up of two waveforms," Convertino said. "The first waveform called the ejected wave is caused by the blood leaving the heart, and the second wave called the 'reflected' wave is caused by the blood being reflected off the arteries back to the heart. These events happen so quickly that the two pressure waves are merged so they look like a single waveform."
Convertino explained that researchers now have the capability to measure features of each arterial waveform that reflect the sum of all mechanisms of compensation that affect the heart. According to Convertino, this measurement is called the 'compensatory' reserve.
The FDA uses the 510(K) premarket submission to ensure that a medical device is safe for use on patients and can then be made commercially available.
"No one has done this before, but we're pretty confident that we can meet FDA requirements," Convertino said.
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.


