USAMMDA Partnership for Pain Relief Tablet
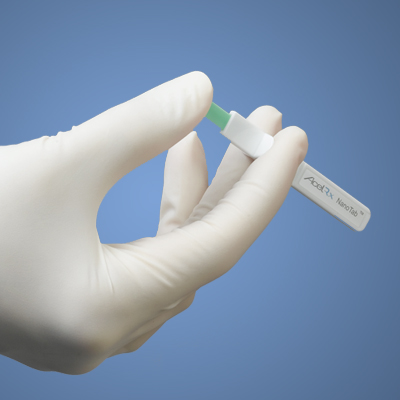
The U.S. Army Medical Materiel Development Activity entered into a contract with AcelRx Pharmaceuticals, Inc., for the partial funding and manufacturing of the company's Phase 3 clinical trial of Sufentanil NanoTab®. The tablet in development is for the treatment of moderate to severe acute pain.
Providing pain relief to wounded service members at point of injury is critical. Sometimes it is the point of injury that makes administering medication difficult. Sufentanil NanoTab® is a small pill administered under a patient's tongue from a pre-filled single-dose applicator. The pill dissolves under the patient's tongue administering faster absorption of the medicine. The cheek and the area under the tongue have a lot of capillaries, or tiny blood vessels, and this means that the medication can be absorbed directly into the bloodstream without needing to go through the digestive system.
According to Andrea Atkinson, product manager for the USAMMDA Pharmaceutical Systems Project Management Office, the current pain management product in the 68W Medical Equipment Set is the outdated 10 mg morphine sulfate auto-injector.
"This drug and means of administration are no longer the accepted standard and have been found in multiple cases of treatment of severe wounds to be ineffective," said Atkinson. "The fielding of the Sufentanil NanoTab® will have an enormous impact on the healthcare to our Soldiers across the full range of military operations by providing a fast-acting, easily dispensed, Sufentanil-based, sublingual pain management product."
The Phase 3 clinical trial will assist with moving the product closer to submission of a New Drug Application and finally U.S. Food and Drug Administration approval.
Atkinson explained that "even though the largest market for this product is the commercial market (hospitals, ambulatory services, etc.), USAMMDA is working with combat medics to ensure that the product design, storage, packaging and so on is acceptable for military use."
FDA approval is anticipated for fiscal year 2018.
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.


