USAMRDC Supports Development of Wearable Head Trauma Diagnostic Device

FORT DETRICK, Md. — A new invention developed with the support of the U.S. Army Medical Research and Development Command's Medical Technology Transfer Office could someday provide medics and first responders with a portable, easy-to-use means of quickly diagnosing and constantly monitoring head injuries, reducing the risk of permanent injury and fatality.
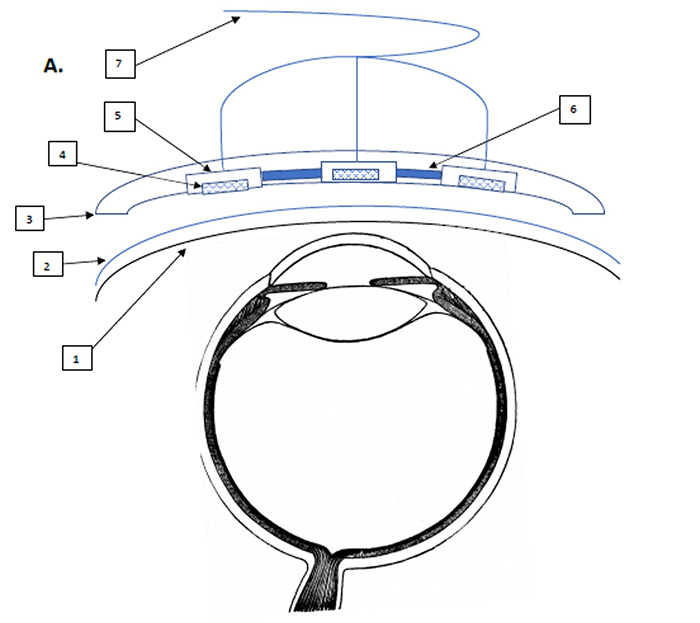
The wearable ultrasound intracranial pressure monitor, invented by Col. Tyler Harris of Womack Army Medical Center and Maj. Jason Perez of Irwin Army Community Hospital, uses multiple small high-frequency sound wave emitters attached to a flexible adhesive strip placed on a patient's closed eyelid. The emitters send out short ultrasonic pulses that measure the diameter of the optic nerve sheath at the back of the eyeball, which has been shown to expand in response to increasing pressure within the skull, thus providing doctors with a useful gauge of the severity of the injury.
Significant head trauma is a complicating injury that occurs in at least 30% of traumatic deaths. Prolonged exposure to elevated pressure inside the skull caused by head trauma can cause strokes, seizures, neurological damage and even death, so immediate diagnosis is essential for a patient's survival and full recovery. Medical professionals already use ultrasound devices to help gauge the severity of intracranial pressure; however, they require skilled technicians to operate them, and they cannot provide constant monitoring of the patient's condition autonomously.
It was those drawbacks that inspired the search for a better alternative during the closing days of the war in Afghanistan.
In August 2021, as U.S. and NATO troops were withdrawing from Afghanistan, Harris and Perez were in Kandahar serving on a 10-person split surgical team assisting in the closure of forward operating bases when they learned that the senior leader of a local organization had a young son with a life-threatening head injury. The team was able to secure a place for the boy on a flight to Bagram Airfield, where neurosurgeons at Craig Joint Theater Hospital could care for him.
Perez, the team's anesthesia provider, used a handheld ultrasound device to determine that the boy's condition was worsening. However, the team could not afford to spare Perez to travel with the boy, and as a result his condition could not be monitored during the flight. Sadly, upon his arrival at Craig the boy was declared brain dead, and his body was subsequently flown back to his family.
"That was a traumatic experience for the team, and it left us with some questions," recalls Harris. "Why didn't we have some kind of capability to monitor patients more consistently in prolonged field care situations or austere conditions?"
Harris and Perez began discussing what such a device would look like while they were still in Afghanistan, and contacted Womack's Clinical Investigation Department to inform them that they might have a patentable idea. The department, in turn, submitted their notes and preliminary sketches to MRDC's Medical Technology Transfer Office, which coordinates intellectual property licensing to help DOD inventors successfully commercialize their products for the Warfighter.
MTT uses an award-winning process it developed called Assistive Technology Transfer, or AT2, which involves working with inventors to systematically mature and de-risk their biomedical technologies to the point that they are ready for licensing by commercial partners. The AT2 process ensures that the first-generation products that reach the Warfighter are mature and military ready. Ron Marchessault, one of MTT's experienced licensing officers, helped Harris and Perez apply for a provisional patent and is working with them to identify and apply for federal funding, including from MRDC's Congressionally Directed Medical Research Programs and the Telemedicine and Advanced Technology Research Center's Advanced Medical Technology Initiative.
The inventors collaborated with a biomedical development company that constructed a basic prototype of the device using parts from a dismantled commercially available handheld ultrasound scanner, with the help of funds provided by Womack's Clinical Investigation Department. Using the prototype to conduct proof-of-concept experiments using a model eyeball and optic nerve sheath, Harris found that the device produced an average measurement that was within 5% of the physical diameter of the sheath, a highly accurate result that validated the invention's premise.
Harris presented these results at the March 2024 State of the Technology Meeting on Neurotrauma Diagnosis, Monitoring and Assessment in Washington, DC, hosted by USAMRDC's Combat Casualty Care Research Program, the Biomedical Advanced Research and Development Authority and the Medical Technology Enterprises Consortium.
"There was definitely interest in it," says Harris. "Obviously folks want to see clinical data, but the potential was definitely recognized, and several folks expressed significant interest in the idea."
Harris says that a wearable ultrasound intracranial pressure monitor could have a wide range of applications in addition to the military, including intensive care units and first responders as well as highly specialized fields such as sports medicine and mountain rescue.
"There's a lot of up-and-coming technology designed to help people manage head trauma, but most of it is still in the early research phase or undergoing clinical trials, and the products are still 10 or 15 years down the road," explains Harris. "With the right funding and the right development team, this device should be relatively easy to transition quickly into the hands of the people who need it."
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.


