USAMRDC Pivots, Attacks in Evolving Virus Response Role
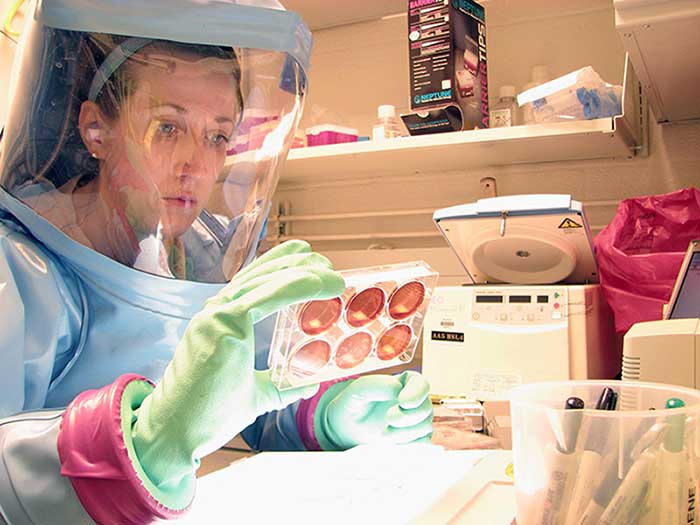
Since the federal government launched its efforts to counteract the spread of the COVID-19 pandemic, the U.S. Army Medical Research and Development Command (USAMRDC) has been a key player in investigating and developing the countermeasures required to both attack and neutralize the virus. Utilizing a 'whole-of-government' approach complemented by a notable history of goal-oriented teamwork and unique research capabilities, USAMRDC is covering a substantial amount of scientific territory – across laboratories located in Maryland, Texas, Alabama, Massachusetts, Thailand, and beyond – in its efforts to prevent, detect and treat COVID-19.
At USAMRDC's U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) scientists have been working to develop countermeasures for the virus since the inception of the federal response. The Institute is noted for its positioning at the forefront of biodefense therapeutics; including the treatments mAB114 and ZMapp, which were used during the West African Ebola virus disease (EVD) outbreak in 2014 and the 2018-2020 EVD outbreak in the Democratic Republic of Congo. Additionally, USAMRIID has both the trained personnel and the specialized Biosafety Level 3 containment laboratories required to safely handle SARS-CoV-2 virus, which is the cause of COVID-19 infection.
Specifically, USAMRIID is pursuing several countermeasure possibilities, including monoclonal antibodies and evaluating convalescent plasma from COVID-19 survivors. The Institute recently received samples from New York to evaluate the ability of the antibodies in such recovered patient plasma to neutralize the SARS-CoV-2 virus. The Institute has additionally generated large quantities of authentic, well-characterized virus for use in developing multiple diagnostic assays and studies assessing the effectiveness of COVID-19 medical countermeasures. Also of note, USAMRIID has provided more than 30 thousand COVID-19 surveillance tests to network laboratories around the world.
Similarly, scientists at USAMRDC's Walter Reed Army Institute of Research (WRAIR) began developing several vaccine prototypes utilizing a previously-tested platform back in January. These prototypes are currently being tested in a small animal models to identify those that induce the strongest immune response, with the most promising prototype subsequently being shipped to USAMRIID for large animal efficacy testing (with human testing being performed at WRAIR). "We have been working on this since the beginning of the outbreak," said Dr. Kayvon Modjarrad, director of WRAIR's Emerging Infectious Diseases Branch. "If we hadn't done that, we'd be weeks behind."
WRAIR's historical footprint in the world of infectious diseases is also significant; the institute has participated in the development of every malaria prevention drug approved by the U.S. Food and Drug Administration (FDA), including – and most recently – tafenoquine in 2018. While USAMRDC scientists hope to have a COVID-19 vaccine available within 12-18 months, they are also utilizing their extensive laboratory capabilities to rapidly advance antiviral treatments.

In a collaborative effort, investigators with both USAMRIID and WRAIR are implementing a screening-based approach to find repurposed drugs for SARS-CoV-2 in the near-term, as well as broad-spectrum drugs that target coronaviruses in general in the long-term. Such work leverages both USAMRIID's biology expertise and WRAIR's chemistry expertise, while also employing a broad network of industrial, academic, non-profit, and U.S. government collaborators. An additional collaboration between both entities involves WRAIR working with a partner to use artificial intelligence (AI) to screen millions of compounds for activity against COVID-19, with animal studies of the most promising studies being performed at USAMRIID.
USAMRDC laboratories are also working to improve the ability to detect COVID-19 infection in Service Members and their dependents. WRAIR scientists are working closely with industry partners in order to advance a range of diagnostic options for small, medium, and large military treatment facilities (MTFs). These include high-throughput tests to identify those who are currently infected and those who are no longer infectious. Furthermore, both WRAIR and USAMRIID are developing the ability to serve as surge capacity for existing MTFs; the Armed Forces Research Institute of Medical Sciences (AFRIMS) – a WRAIR overseas research laboratory headquartered in Bangkok, Thailand – is currently performing COVID-19 testing as well.
In a display of the command's unique capabilities, USAMRDC's U.S. Army Aeromedical Research Laboratory (USAARL) performed a rapid-response evaluation of aircrew performance while wearing both an N-95 Respirator mask and various models of other cloth masks to assess the effects of wearing the mask for speech intelligibility, usability, workload, situational awareness and comfort, and to determine which masks are compatible with safe flight. According to the Centers for Disease Control and Prevention (CDC), the N-95 mask filters out at least 95% of airborne particles – including both large and small particles – when properly fitted and donned, making it a more professional grade mask. The lab is also testing several patient isolation units to possibly transport COVID-19 patients on U.S. Army and Army National Guard medical evacuation aircraft.
"These evaluations afforded a team of researchers and pilots the honor of responding to exigent safety needs of the Army aviation community while directly observing the impact of their scientific efforts," said USAARL Auditory and Human Factors Researcher and Deputy Group Director, Warfighter Performance Group LTC Kara Cave. "Oftentimes in research the benefit to aviators comes many months, if not years, after a study. So, the team really enjoyed seeing the rapid benefit to Soldiers. The synergy and dedication among the USAARL team enabled a less than 24-hour response time and helped establish USAARL as a resource for aviation-related performance and safety issues."
Another unique response to the pandemic comes from USAMRDC's Telemedicine & Advanced Technology Research Center (TATRC), which is currently working with the Society for Critical Care Medicine (SCCM), the U.S. Department of Health and Human Services (HHS), the Assistant Secretary for Preparedness & Response (ASPR) and the Federal Emergency Management Agency (FEMA) to support the rapid development and testing of a prototype National Emergency Telecritical Care Network (NETCCN). The NETCCN is a cloud-based, low-resource, stand-alone health information management system designed for the creation and coordination of flexible and extendable "virtual critical care wards." If fully implemented, these wards – which are based on existing cellular communication networks, mobile technologies and cloud computing capabilities – would bring high-quality critical care capability to nearly every bedside in the U.S. regardless of location. TATRC has received over 80 proposals for NETCNN and will soon issue awards to clinical and technical teams to begin prototyping and rapid iterative development.
Further, in an effort spurred by a shortage of critical personal protection equipment for healthcare professionals at Walter Reed National Military Medical Center (WRNMMC), TATRC staffers teamed up to produce more than 250 reusable face shield frames in less than 48 hours via internal 3D printing technology. Nathan Fisher, a research project manager at TATRC, found an open-source design for the masks and teamed up with the USAMMDA prototype shop to print the initial proof-of-concept before mobilizing local volunteers for larger production. Fisher then personally hand-delivered the entire shipment of completed masks to WRNMMC staff within a week.

Meanwhile, the U.S. Army Medical Materiel Development Activity (USAMMDA) has entered into a Cooperative Research and Development Agreement (CRADA) with Gilead Sciences, Inc., to provide Gilead's investigational drug, remdesivir, for the treatment of Department of Defense (DOD) personnel diagnosed with moderate to severe COVID-19. Since then, the drug has been provided to 20 MTFs and several patients have either received or are currently receiving the treatment.
USAMMDA has also contributed via their efforts to support the deployment of four hospital centers and two combat support hospitals; an announcement made in early April. The USAMMDA Warfighter Deployed Medical Systems (WDMS) team has been tasked with filling shortages for deploying units by surveying current equipment, facilitating delivery of key materials and coordinating with other agencies to ensure deploying units have the equipment required to combat the pandemic. "WDMS pulls a report every morning to show the available stock in the warehouses for the Army," says Lindsay Longobardi, Deputy Project Manager for USAMMDA WDMS. "[W]e have to prioritize and fill shortages based on which units are deploying. Since the novel coronavirus is now a pandemic and a National Emergency, it is our top priority to support this mission."
At USAMRDC's U.S. Army Institute of Surgical Research (USAISR), efforts are underway to design a clinical study using antibodies in the blood of people who have survived COVID-19 to treat other infected patients. "Research studies from previous hemorrhagic shock researchers to identify those parts of the blood that confers immunity in patients who have already recovered from the infections are liable to be the fastest and most effective way to treat this deadly virus in severely impacted individuals," said Air Force Col. (Dr.) Erik Weitzel, USAISR Deputy Commander.
In an effort to spur more wide-ranging, collaborative efforts to combat the pandemic, the Command is turning to its partnership with the Medical Technology Enterprise Consortium (MTEC). A nonprofit corporation comprised of hundreds of private, academic and not-for-profit organizations, MTEC is designed to accelerate the translation of medical technologies into solutions focusing chiefly on the health of U.S. military personnel and veterans. An integral part of MTEC's partnership with the DOD – and indeed, an integral part of its efforts as related to the COVID-19 fight – rests within its use of the OTA (or, Other Transaction Agreement), a unique and powerful tool which allows MTEC to operate a solicitation and award process in a more open, transparent, and collaborative manner, tearing down traditional acquisitions barriers restricting government-industry interactions. As part of that process, MTEC is currently working a number of short-turnaround efforts in topic areas related to COVID-19, ultimately seeking to solicit ideas (vis-Ã -vis scientific proposals) and award funding as expeditiously as possible.
The first solicitation released by MTEC was designed to identify novel therapeutic treatments largely focused on repurposed drugs with different – but related – indications; or novel drugs that are ready for human studies. Further, MTEC anticipates releasing additional solicitation announcements in the near future regarding, among other efforts, a wearable diagnostic to detect pre- or early symptomatic COVID-19 infection. Funding for the aforementioned NETCCN was also released through MTEC. Overall, MTEC plans to award more than 50 million dollars as part of the fight against the virus.
"MTEC is proud to be a partner with USAMRDC to accelerate the development of solutions for COVID-19 and to protect and heal those who serve," said Kathy Zolman, MTEC Director of Program Operations.
A focus on wearable systems also plays a large role in the contribution of USAMRDC's U.S. Army Research Institute of Environmental Medicine (USARIEM) to the Command's overall effort. Currently, USARIEM is evaluating the use of its ECTemp (or, Estimated Core Temperature) algorithm – a method developed by USARIEM's Dr. Mark Buller to provide accurate estimates of core body temperature by analyzing heart rate changes over time – to detect key early symptoms and monitor body temperature for fever. Further, USARIEM is also serving as the DOD lead for one of the subgroups under the Mass General Brigham (MGB) Center for COVID Innovation, Direct to Consumer Mobile Health Working Group, which is in turn addressing the monitoring of service providers and patients in disaster scenarios.
With a commitment to ongoing dialogue with thought leaders in the corporate and academic arenas, USAMRDC's New Product Idea (NPI) website – an online tool designed to field potential solutions for any number of military-related gaps and concerns – has received more than 80 inquiries from a variety of vendors and contractors across a broad spectrum efforts and products through the first week of April alone. In fact, due to the high rate of interest, USAMRDC is now working with the Army Application Laboratories (AAL) at the Army Futures Command to conduct an initial assessment of the inquires.
To further help encourage these types of new, innovative efforts to combat COVID-19, USAMRDC's Congressionally Directed Medical Research Programs (CDMRP) recently released several new funding opportunities for virus research. Specifically, CDMRP released four dedicated Program Announcements (PAs) focused directly on COVID-19 and SARS-COV-2; with funding to be routed to topic areas encompassing emerging viral diseases and respiratory health.
While USAMRDC is just one of the DOD entities working to combat COVID-19, the command's ability to access diverse internal resources while also leveraging external partnerships in order to find a solution reveal it to be an integral and intricately-connected group of highly-qualified experts who are ready to respond to one of the greatest challenges many of us have ever seen. Further, the USAMRDC routinely coordinates across the larger DOD to leverage expertise and capabilities to address the growing spectrum of pandemic-related needs such as research and development for medical countermeasures, manufacturing, and more. In short, the USAMRDC response is sprawling, robust, and evolving as needed.
"We are supporting a whole-of-government approach to detect, prevent and treat COVID-19," said Brigadier General Michael J. Talley, Commanding General of USAMRDC and Fort Detrick, Maryland, during a news conference from the Pentagon in March. "We're all working towards a solution, and we want to get it done as quickly as possible."
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.


