Office of Regulated Activities
Ebola Virus Emergency Use Authorizations
The U.S. Food and Drug Administration maintains a web page containing current and terminated Emergency Use Authorizations that make available diagnostic and therapeutic medical devices to diagnose and respond to public health emergencies.
FDA Emergency Use Authorizations
- Healthcare Provider Fact Sheets (PDF)
- Patient Fact Sheets (PDF)
Certificate of Analysis
- EUA Assay Ebola Zaire (PDF)
- EUA Positive Control (PDF)
- RNAseP Positive Control (PDF)
- RNAseP Master Mix (PDF)
- Ordering Information
Reporting Information
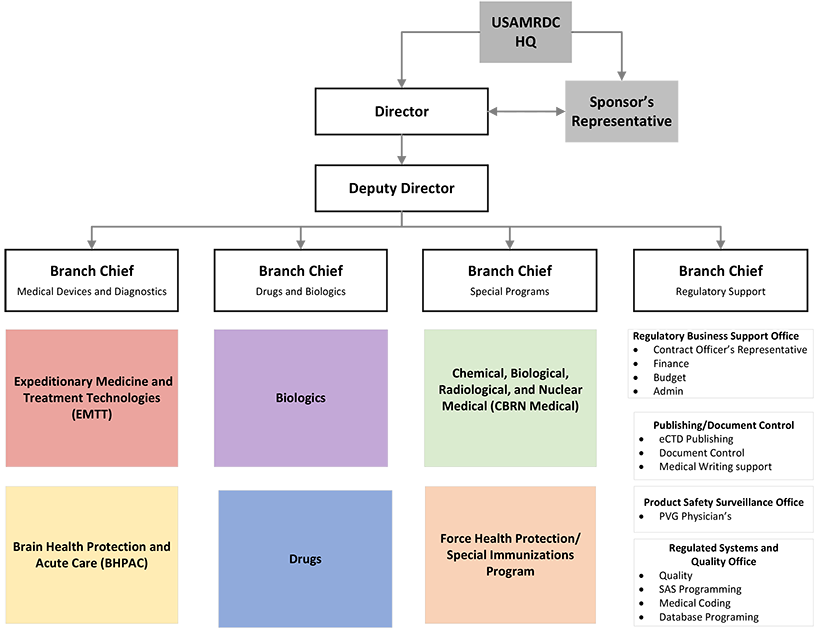
- The Office of Regulated Activities (ORA) is a multidisciplinary team of regulatory affairs, compliance, and clinical support professionals dedicated to supporting the USAMRDC mission of developing Food and Drug Administration regulated medical products for the Warfighter. The ORA provides full-service, oversight, and consultation for regulatory, clinical, non-clinical, manufacturing, data management, biostatistics, product technical, safety monitoring, and medical and regulatory writing support for FDA-regulated drug, biologic, medical device, and combination products.
- Levels of Support
- Full support for The Surgeon General-Department of the Army (TSG-DA) sponsored products and research efforts
- Oversight for all TSG-DA sponsored products contracted out to Contract Research Organizations
- Oversight for contracted product development for non-TSG sponsored products
- Consultation/Advisory Services for non-TSG-DA sponsored activities
- For additional information please visit the ORA SharePoint site.
- The ORA is organized into 3 regulatory branches made up of matrixed teams of regulatory experts spanning all disciplines and 1 regulatory support branch. Regulatory support begins with the initial regulatory strategy and throughout advanced development, to mitigate risk and accelerate the delivery of FDA-Regulated products to Service Members.
Last Modified Date: 23-May-2023
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.